Explore Top Pharmaceutical Products, APIs, Formulations & Intermediates from Verified Suppliers
Paracetamol (Acetaminophen) Injection
Strength:
1000 mg/100 mL (10 mg/mL)
Form: Injectable solution
Reference Brands: Ofirmev
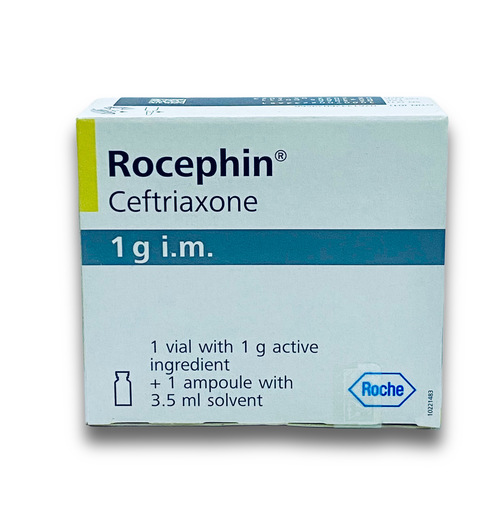
View More Get EnquiryCeftriaxone Injection
Strength:
250 mg, 1 g, and 2 g
Form: Lyophilized powder/ Prefilled syringe
Reference Brands: Rocephin
View More Get EnquiryItraconazole Pellets
Strength:
100 mg or 200 mg
Form: Pellets
Reference Brands: Sporanox
View More Get EnquiryLansoprazole Pellets
Strength:
15 mg and 30 mg
Form: Pellets
Reference Brands: Prevacid
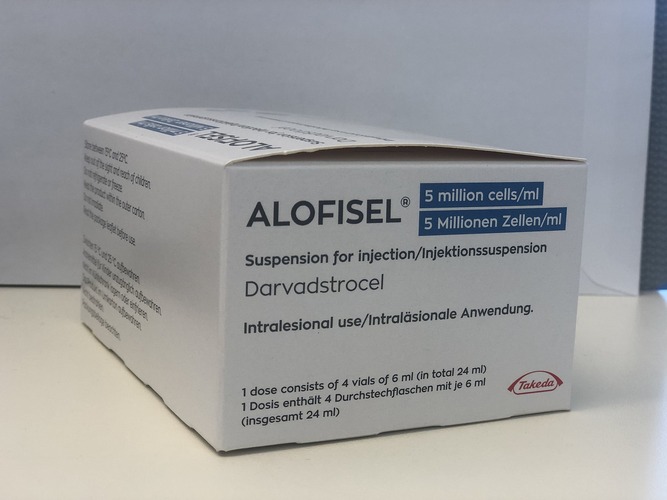
View More Get EnquiryDarleukin Injection
Strength:
A standardized dose of 120 million adipose-derived stem cells per injection
Form: Intralesional injection
Reference Brands: Alofisel(US & EU)
View More Get EnquiryEtranacogene Dezaparvovec. Infusion
Strength:
Single infusion product designed to produce sustained factor IX levels in hemophilia B patients, dosage individualized based on patient weight
Form: Intravenous infusion
Reference Brands: Hemgenix (US & EU)
View More Get EnquiryElivaldogene Autotemcel Infusion
Strength:
A personalized gene therapy designed to halt or slow ALD progression, dosed based on weight, administered as a single infusion in specialized treatment centers
Form: Intravenous infusion
Reference Brands: Skysona(US & EU)
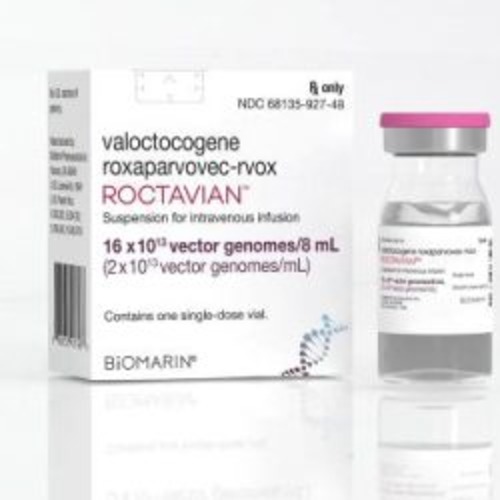
View More Get EnquiryValoctocogene Roxaparvovec Infusion
Strength:
A single-dose gene therapy designed to increase factor VIII production in hemophilia A patients
Form: Intravenous infusion
Reference Brands: Roctavian(US & EU)
View More Get EnquiryVoretigene Neparvovec Infusion
Strength:
Adeno-associated virus (AAV) vector administered via subretinal injection
Form: Subretinal injection
Reference Brands: Luxturna(US & EU)
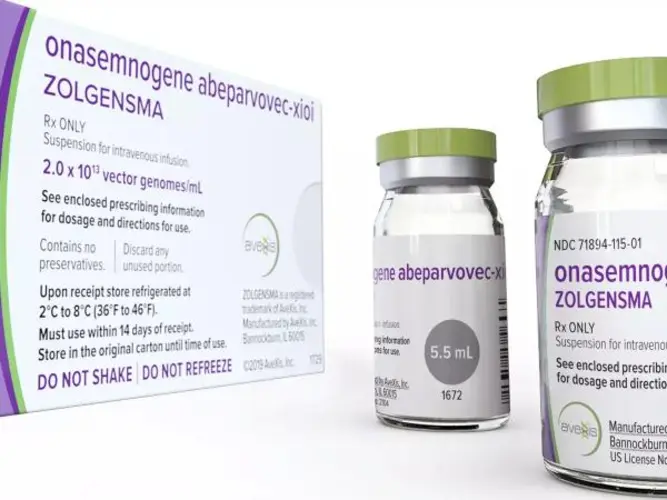
View More Get EnquiryOnasemnogene Abeparvovec Injectable
Strength:
Single-dose IV infusion providing 6 x 10^13 vector genomes (vg) per patient
Form: Injectable gene therapy
Reference Brands: Zolgensma(US & EU)
View More Get EnquiryIdecabtagene Vicleucel Infusion
Strength:
Approved for relapsed/refractory multiple myeloma, with dosing based on patient weight, delivering a personalized, targeted therapy to eliminate cancer cells
Form: IV Infusion
Reference Brands: Abecma(US & EU)
View More Get EnquiryLisocabtagene Maraleucel Infusion
Strength:
Approved for relapsed/refractory large B-cell lymphoma, with a standard dose of 100 million CAR T cells per infusion
Form: IV Infusion
Reference Brands: Breyanzi (US & EU)
View More Get EnquiryTisagenlecleucel Infusion
Strength:
Single infusion, approved for relapsed/refractory B-cell precursor acute lymphoblastic leukemia (ALL) and diffuse large B-cell lymphoma (DLBCL)
Form: IV Infusion
Reference Brands: Kymriah(US & EU)
View More Get EnquiryInsulin‑Aspart‑Szjj
Strength:
100 units/mL
Form: SQ Injection
Reference Brands: NovoLog; Merilog is biosimilar to NovoLog.
View More Get EnquiryEculizumab‑Aeeb
Strength:
300 mg/20 mL (IV infusion),
Form: Injection for intravenous infusion
Reference Brands: Soliris, Bkemv- Biosimilar of Soliris, Epysqli- Biosimilar of Soliris
View More Get EnquiryOmalizumab‑Igec
Strength:
75 mg/0.5 mL and 150 mg/mL
Form: Pre filled syringe
Reference Brands: Xolair, Omlyclo - a Biosimilar to Xolair
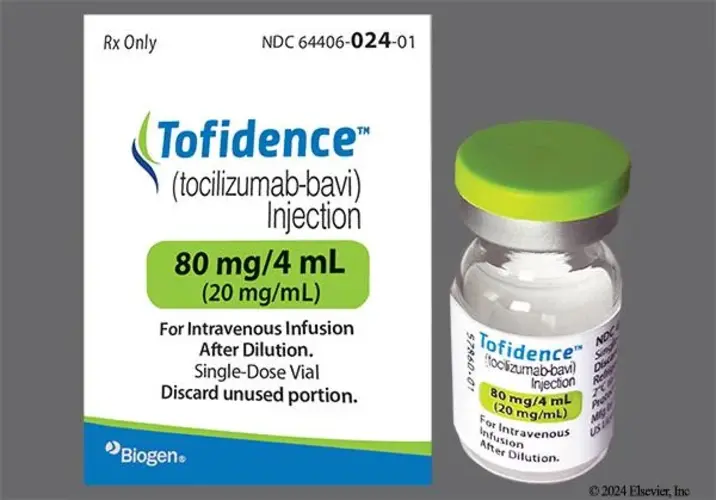
View More Get EnquiryTocilizumab-Bavi
Strength:
80 mg/4ml
Form: Intravenous infusion
Reference Brands: Actemra; Tofidence - biosimilar to Actemra (tocilizumab).
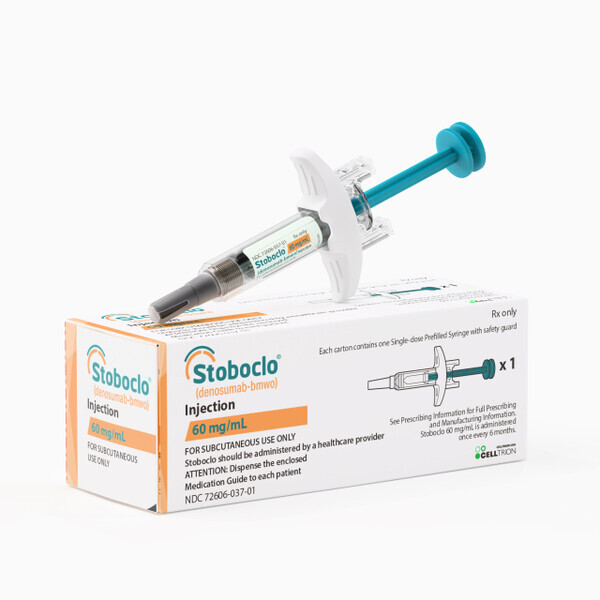
View More Get EnquiryDenosumab(Others Approved)
Strength:
60 mg, 120 mg
Form: Subcutaneous injection
Reference Brands: Xgeva(US & EU) Ospomyv, Xbryk, Merilog, Stoboclo, Zadenvi, Enwylma, Denbrayce, Izamby - biosimilar to Xgeva
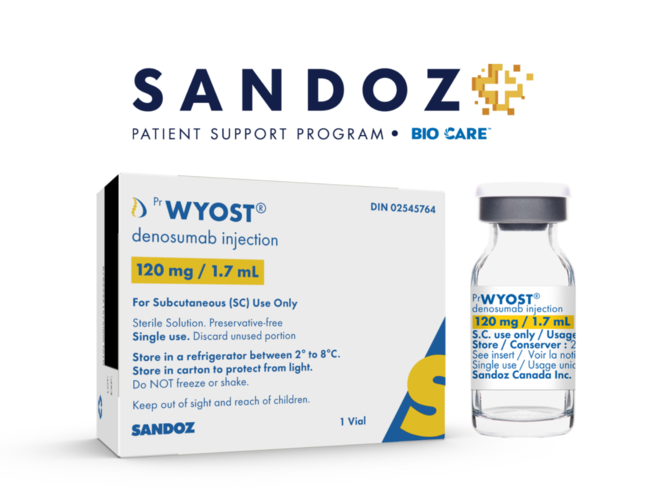
View More Get EnquiryDenosumab
Strength:
subcutaneous injection
Form: Subcutaneous injection
Reference Brands: Xgeva; Wyost - biosimilar to Xgeva
View More Get EnquiryDenosumab-Bbdz
Strength:
60 mg, 120 mg
Form: Injection for subcutaneous use
Reference Brands: Prolia(US & EU); Jubbonti - biosimilar to Prolia
View More Get Enquiry