Explore Top Pharmaceutical Products, APIs, Formulations & Intermediates from Verified Suppliers
Vimseltinib
Strength:
14 mg; 20 mg; 30 mg
Form: Capsules
Reference Brands: Romvimza
View More Get EnquiryMetformin Crystals/Powder
Strength:
Supplied as high-purity crystals in 99%
Form: Crystals/Powder
Reference Brands: Generic metformin
View More Get EnquiryParacetamol (Acetaminophen) Granules
Strength:
High-purity granules or powder with concentrations usually 50% or higher
Form: Granules for reconstitution; Bulk powder
Reference Brands: Generic formulations
View More Get EnquiryOmeprazole Pellets
Strength:
Similar strengths (10 mg, 20 mg, 40 mg); pellets for enteric coating to optimize drug stability and absorption
Form: Pellets
Reference Brands: Prilosec(US)
View More Get EnquiryAxicabtagene Ciloleucel Infusion
Strength:
Single infusion treatment approved for relapsed/refractory large B-cell lymphoma, with dosing based on patient weight
Form: IV Infusion
Reference Brands: Yescarta (US & EU)
View More Get EnquiryAflibercept‑Ayyh
Strength:
2 mg/0.05 mL
Form: Intravitreal injection (liquid solution)
Reference Brands: Eylea(US & EU); Pavblu - biosimilar to biosimilar to Eylea
View More Get EnquiryUstekinumab‑Aekn
Strength:
Intravenous Infusion(130 mg/26 mL), Subcutaneous injection (pre-filled syringes, pens)
Form: Subcutaneous injection (pre-filled syringes, pens); Intravenous Infusion
Reference Brands: Stelara(US & EU); Selarsdi- biosimilar of Stelara
View More Get EnquiryMrna-Based Vaccines
Strength:
30 µg and 100 µg
Form: Injectable suspension
Reference Brands: Comirnaty, Spikevax(US)
View More Get EnquiryPcv (Pneumococcal Conjugate Vaccine)
Strength:
0.5 mL
Form: Vial doses, Pre-filled syringes
Reference Brands: Prevnar 13(US)
View More Get EnquiryAmoxicillin Trihydrate Capsule
Strength:
250 mg, 500 mg, 875 mg, 1000 mg
Form: Tablets/Capsules/Powder for suspension
Reference Brands: Amoxil(US)
View More Get EnquirySemaglutide Injectable
Strength:
0,25 mg - 2.4 mg per injection for weight management
Form: Injection(SQ)
Reference Brands: Wegovy(US & EU)
View More Get EnquiryHyaluronic Acid Injectable
Strength:
1 mL syringes
Form: Injectable Solutions
Reference Brands: Juvederm, Restylane, Belotero(US)
View More Get EnquiryMelatonin Tablet
Strength:
1 mg, 3 mg, 5 mg, or 10 mg
Form: Tablets
Reference Brands: Natrol, REMfresh, Nature’s Way(US)
View More Get EnquiryCalcium Citrate/Malate Capsules And Tablets:
Strength:
250 mg, 500 mg, and 1000 mg
Form: Capsules and Tablets:
Reference Brands: NOW Foods, Nature’s Way, Solgar:(US)
View More Get EnquiryPrednisolone Tablets
Strength:
5 mg, 20 mg, 50 mg
Form: Tablets
Reference Brands: Generics & generic brands available in EU &US
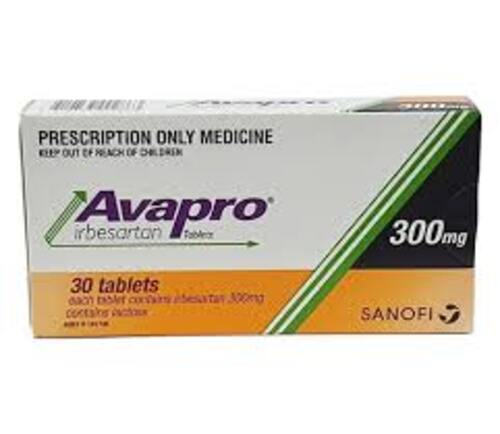
View More Get EnquiryIrbesartan Tablets
Strength:
75 mg, 150 mg, 300 mg
Form: Tablets
Reference Brands: Avapro(Us & EU)
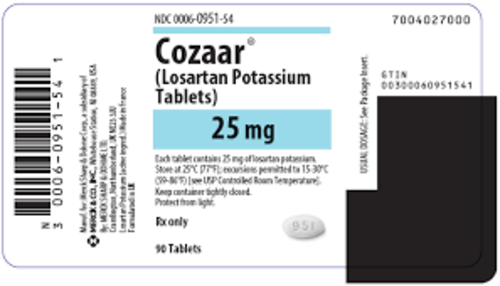
View More Get EnquiryLosartan Potassium Tablet
Strength:
25 mg, 50 mg, 100 mg
Form: Tablet
Reference Brands: Cozaar(US & EU)
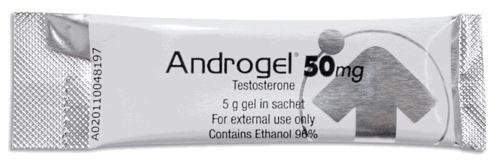
View More Get EnquiryTestosterone Gel
Strength:
20.25 mg, 40.5 mg, or 50 mg
Form: topical application/ gel
Reference Brands: AndroGel, Testim, Axiron(US)
View More Get EnquiryVitamin B12 (Cyanocobalamin / Methylcobalamin) – Tablets
Strength:
500 mcg, 1000 mcg, 2000 mcg (2 mg)
Form: Tablet
Reference Brands: Nature Made, Solgar, NOW Foods(US); Femibion, Vitafol, Solgar(EU)
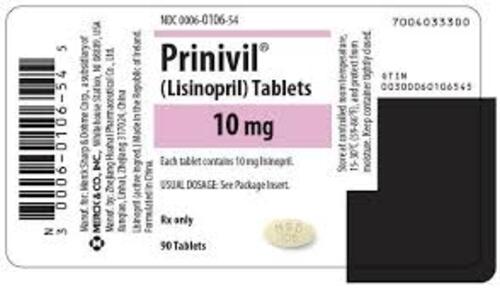
View More Get EnquiryLisinopril Tablets
Strength:
2.5 mg, 5 mg, 10 mg, 20 mg, 40 mg
Form: Tablet
Reference Brands: Prinivil, Zestril(US & EU)
View More Get Enquiry