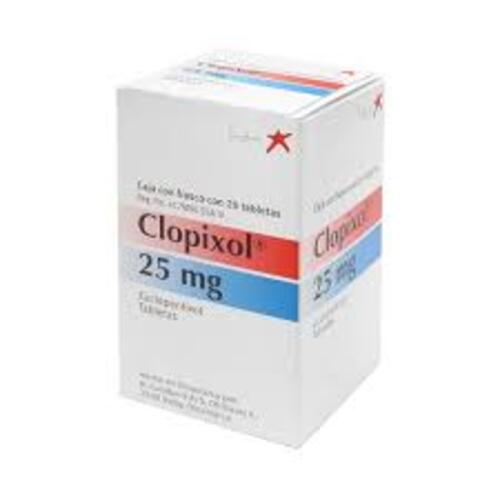
Zuclopenthixol tablets
Form: Oral Tablets
Strength: 10 mg, 25 mg, 40 mg
Reference Brands: Clopixol®(EU)
Category: Antipsychotropic Drugs
Zuclopenthixol tablets, used for managing schizophrenia and chronic psychoses, are not FDA-approved in the United States but are authorized in the European Union under the brand Clopixol. EU regulations require GMP compliance, pharmacovigilance systems, and bioequivalence data for registration. U.S. market entry would require a 505(b)(2) or ANDA submission with complete CMC, clinical, and labeling documentation. Regulatory focus includes psychiatric safety profiling and consistent release characteristics. For dossier-ready Zuclopenthixol tablets and to explore other available forms such as IM injections and depot formulations, visit Pharmatradz.com — your trusted platform for global pharmaceutical sourcing.
The Trade Mark owner has been correctly identified under the heading Manufacturer / TM Owner as required by the Act.
Related Products
Risperidone Long-Acting Injection
Strength: 12.5 mg, 25 mg, 37.5 mg, 50 mg vials/syringes
Form: Long-Acting Injection
Reference Brands: Risperdal Consta(US &EU)
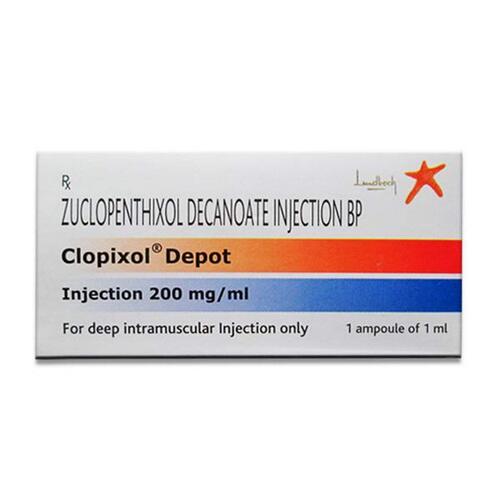
View More Get EnquiryZuclopenthixol ong-acting IM Injection (Depot)
Strength: 200 mg/mL, 500 mg/mL
Form: Long-acting IM Injection (Depot)
Reference Brands: Clopixol Depot®(US)
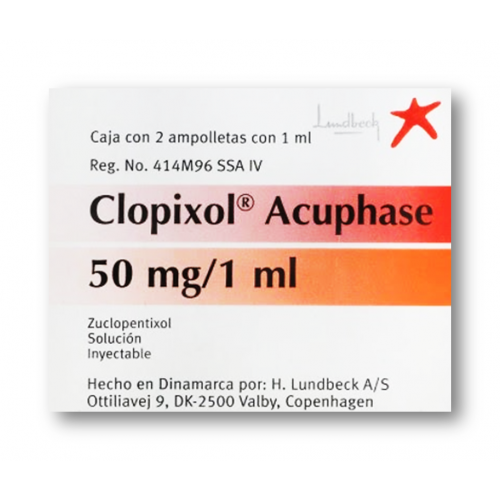
View More Get EnquiryZuclopenthixol Short-acting Intramuscular Injection
Strength: 50 mg/mL
Form: Short-acting Intramuscular Injection
Reference Brands: Clopixol-Acuphase®
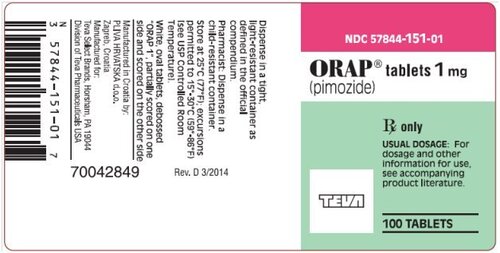
View More Get EnquiryPimozide Tablets
Strength: 1 mg, 2 mg, 4 mg
Form: Oral Tablets
Reference Brands: Orap®(US & EU)
View More Get Enquiry