Metformin + Vildagliptin Tablets
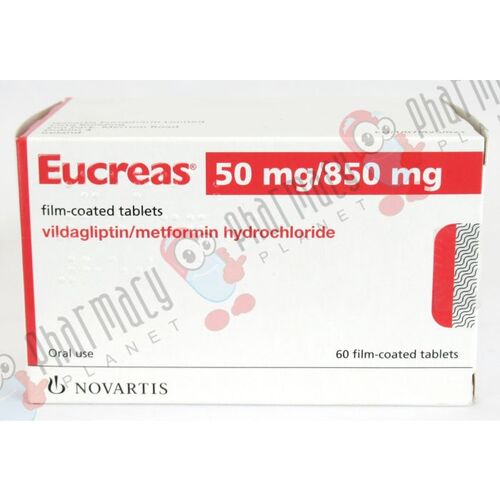
Form: Tablets
Strength: 50/500 mg/850mg/1000mg
Reference Brands: Eucreas®(EU)
Category: Diabetes
EMA (EU): Approved as Eucreas® (Novartis) in fixed-dose combinations (50mg vildagliptin + 500/850/1000mg metformin). Key requirements: Boxed warnings: None, but pancreatitis risk requires discontinuation if suspected Hepatic monitoring: Mandatory (rare hepatitis reports) Renal restrictions: Contraindicated if eGFR <30; dose adjustment for eGFR 30-60 Storage: <30°C (protect from moisture) FDA (US): Not approved - Vildagliptin development discontinued in 2009 due to hepatotoxicity concerns in Phase III trials. Shared Requirements: Lactic acidosis warnings (metformin) Pregnancy Category B (avoid unless essential). For regulatory support: PharmaTradz.com
The Trade Mark owner has been correctly identified under the heading Manufacturer / TM Owner as required by the Act.
Related Products
Premixed Insulins (70/30, 50/50) Injectable
Strength: U-100
Form: Vials, pens
Reference Brands: Humulin, Novolin(US); Mixtard, Humulin, Insuman(EU)
View Details Get EnquiryInsulin Lispro (Humalog) injectable
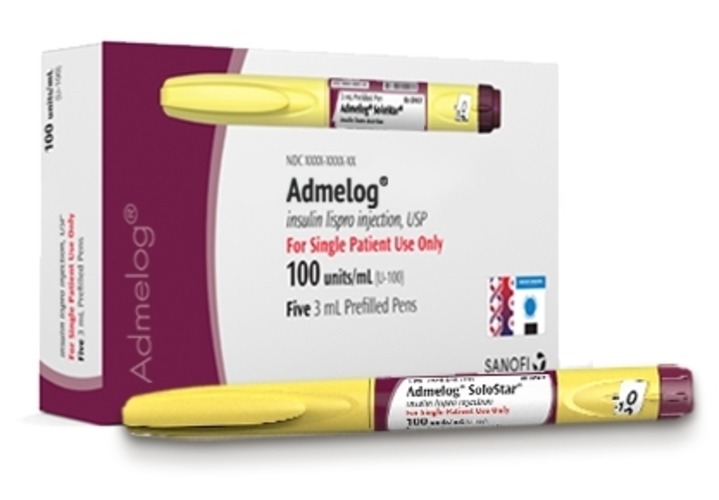
Strength: U-100, U-200
Form: Vials/pens
Reference Brands: Humalog, Admelog, Fiasp(US); Humalog, Fiasp(EU)
View Details Get EnquiryInsulin Aspart (Novolog) injectable
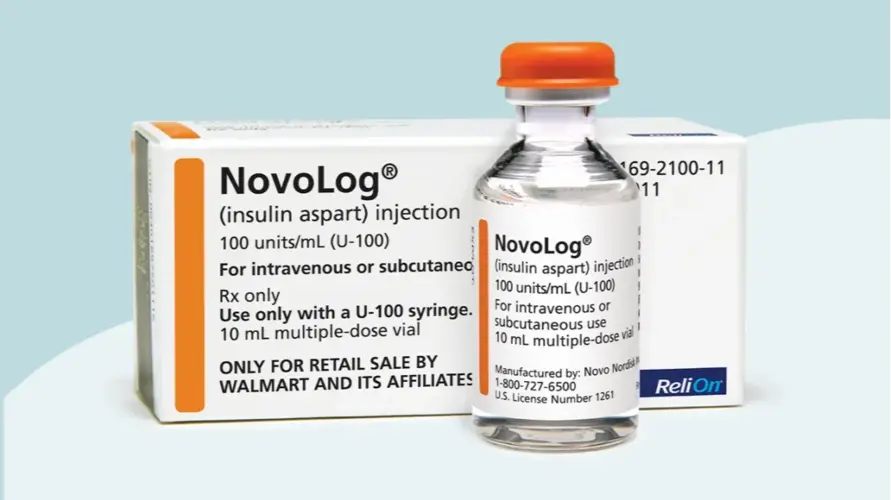
Strength: U-100, U-200
Form: Vials/pens
Reference Brands: Novolog, Fiasp(US &EU)
View Details Get EnquiryInsulin Glargine (Lantus) injectable
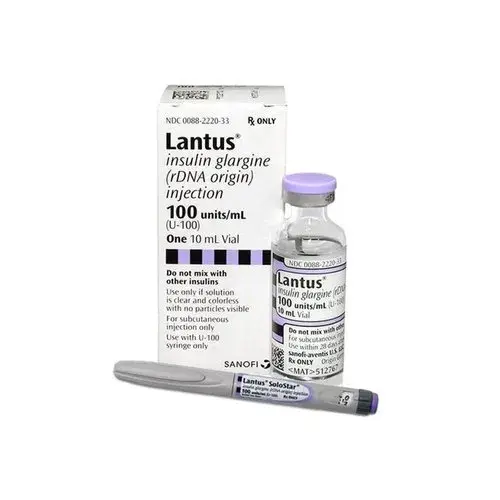
Strength: U-100, U-300
Form: Vials/pens
Reference Brands: Lantus, Basaglar, Toujeo(US); Lantus, Toujeo(EU)
View Details Get Enquiry