Fresh Frozen Plasma (FFP) powder for reconstitution or liquid
Form: liquid units or lyophilized (freeze-dried) powder
Strength: available in 200-250 mL units
Reference Brands: No specific brands
Category: Plasma Products
Frozen Plasma (FFP), used for coagulation correction and volume resuscitation, is regulated in the US by the FDA and in the EU via EMA approval pathways. Regulatory approval requires a comprehensive dossier including manufacturing standards, donor screening, safety, efficacy, and pharmacovigilance plans. In the US, FDA review assesses clinical and quality data, while EMA ensures compliance with regional safety and quality standards. For guidance on dossier preparation, regulatory pathways, and market access, visit PharmaTradz. Ensuring regulatory compliance is essential for the safe, effective, and timely approval of FFP products worldwide, supporting optimal patient care in critical bleeding and coagulation disorders.
The Trade Mark owner has been correctly identified under the heading Manufacturer / TM Owner as required by the Act.
Related Products
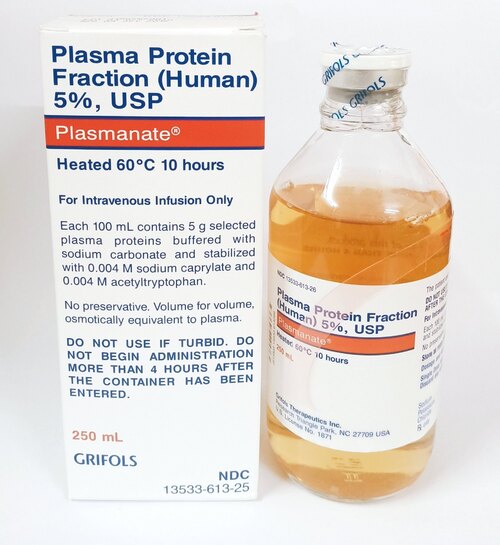
Plasma Protein Fraction (PPF) powder
Strength: 50 mL, 250 mL, or 500 mL
Form: Lyophilized Powder or Liquid
Reference Brands: Plasmanate (US), Octaplas LG, Biotest Plasma (EU)
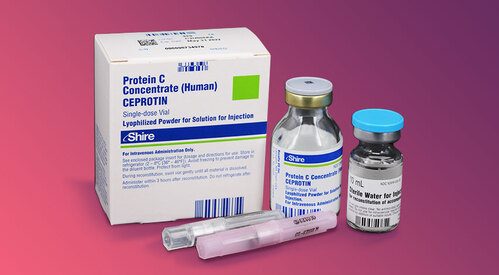
View More Get EnquiryProtein C Concentrate powder
Strength: 5,000 IU/vial
Form: Lyophilized Powder
Reference Brands: Ceprotin(US)
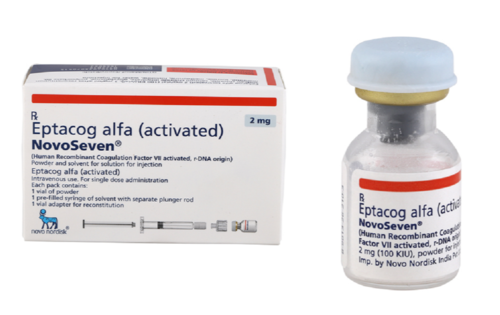
View More Get EnquiryProthrombin Complex Concentrate (PCC) Injectable concentrates
Strength: 1,200 IU/vial
Form: Injection
Reference Brands: NovoSeven, AryoSeven, BS-505(US)
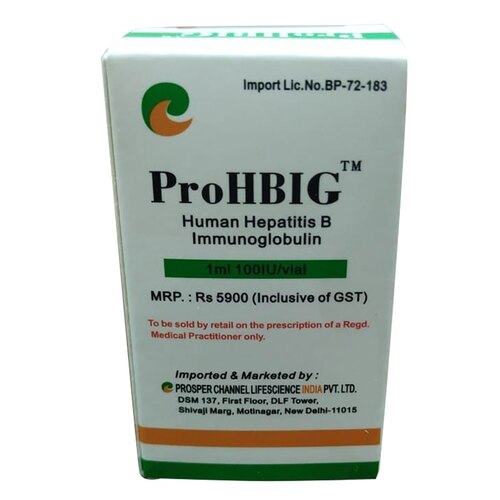
View More Get EnquiryHyperimmune Globulins Intravenous (IV) or Subcutaneous (SC)
Strength: 100 mg/mL or 50 mg/mL
Form: Intravenous (IV) or Subcutaneous (SC) Solution
Reference Brands: Tetanus IG (TIG), Varicella Zoster IG, Cytogam,RhoGAM,Hepatitis B Immune Globulin (HBIG)(US)
View More Get Enquiry