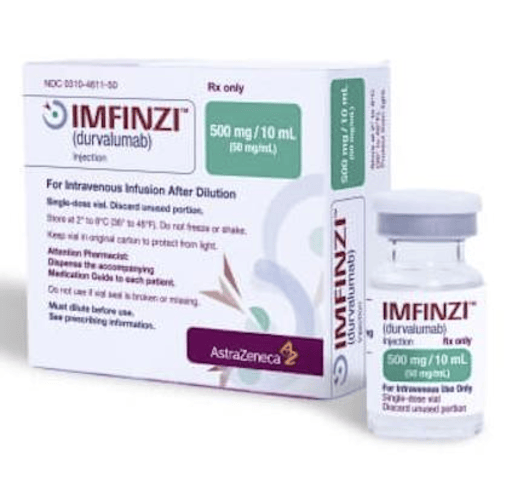
Durvalumab
Form: Injection
Strength: 120 mg/4 mL, 500 mg/10 mL
Reference Brands: Imfinzi® (US & EU)
Category: Oncology Cancer Care
Durvalumab (Imfinzi®) is a PD-L1 immune checkpoint inhibitor indicated for urothelial carcinoma and non-small cell lung cancer. Provided as sterile intravenous infusion vials (120 mg/4 mL and 500 mg/10 mL), Durvalumab is manufactured under EU-GMP and USFDA standards. Available for B2B supply, contract manufacturing, and licensing, it supports hospital tenders and oncology distributors with full regulatory documentation. This monoclonal antibody enhances anti-tumor immunity by blocking PD-L1, making it a critical drug in immuno-oncology portfolios across the US and European markets.
The Trade Mark owner has been correctly identified under the heading Manufacturer / TM Owner as required by the Act.
Related Products
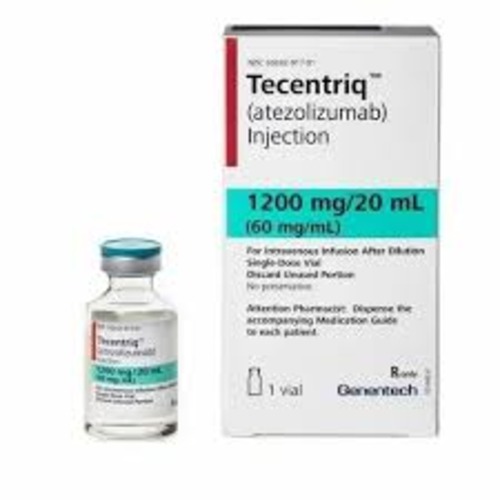
Atezolizumab
Strength: 1200 mg/20 mL
Form: Intravenous infusion
Reference Brands: Tecentriq® (EU & US)
View More Get EnquirySunitinib
Strength: 12.5 mg, 25 mg, 37.5 mg, 50 mg
Form: Tablet
Reference Brands: Sutent® (EU & US)
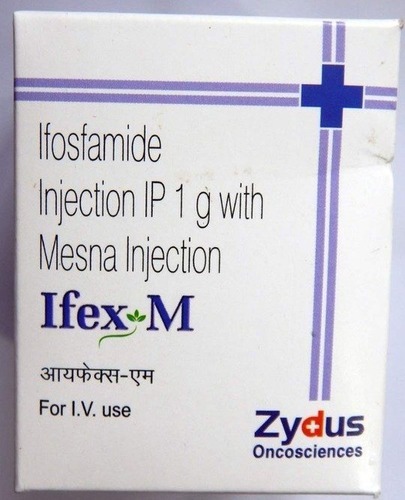
View More Get EnquiryIfosfamide
Strength: 1 g/vial,2 g/vial, 3 g/vial
Form: Injection
Reference Brands: Ifex® (US), Holoxan® (EU)
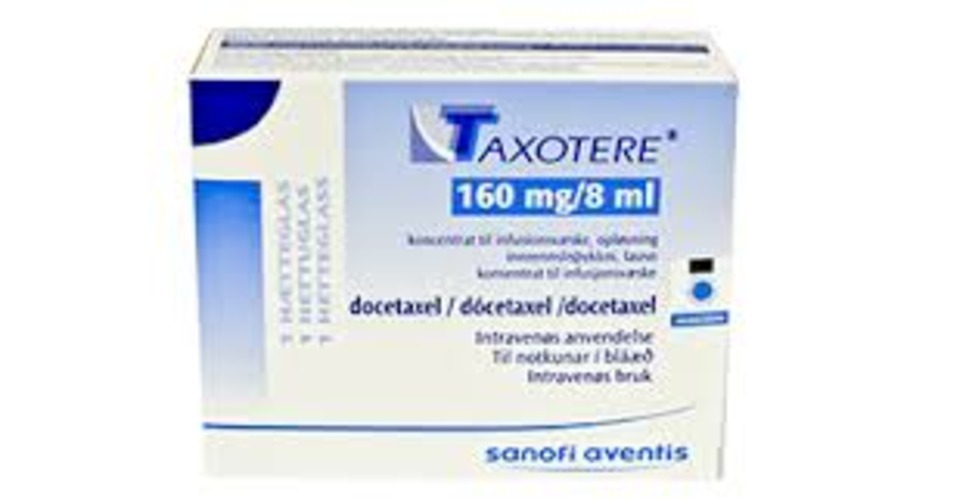
View More Get EnquiryDocetaxel
Strength: 20 mg/0.5 mL, 80 mg/2 mL, 160 mg/4 mL
Form: Injection
Reference Brands: Taxotere® (US & EU), Docefrez® (EU)
View More Get Enquiry