How to Source Nasal Cannulas and Oxygen Masks Medical Device for Clinical Use
Nasal Cannulas and Oxygen Masks (Nasal cannulas: Deliver low-flow oxygen via prongs inserted into nostrils, Oxygen masks: Cover nose and mouth for higher flow rates, Nasal cannulas typically deliver 1-6 L/min; masks provide 6-15 L/min, available in various sizes and materials) is classified under Medical Devices. It is therapeutically aligned with reference brands such as Puritan Bennett, Salem Silk, Hartmann, Linde. This guide highlights key sourcing factors buyers should consider when procuring high-quality Nasal Cannulas and Oxygen Masks for formulation, R&D, or bulk manufacturing.
Product Overview:
Nasal cannulas and oxygen masks deliver supplemental oxygen directly to the respiratory system. Cannulas provide low-flow oxygen, ideal for long-term use, while masks offer higher flow rates for acute needs. Benefits include improved oxygenation, enhanced breathing, relief from hypoxia, and supporting respiratory stability in medical and veterinary care.
Nasal cannulas and oxygen masks are regulated in both the EU and US, with dossiers demonstrating saf...
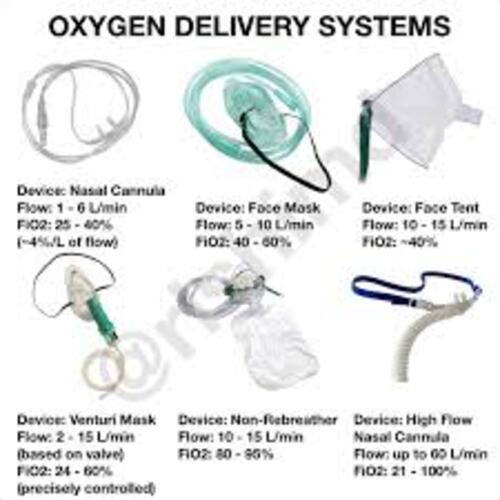 Nasal Cannulas and Oxygen Masks Medical Device for Clinical Use
Nasal Cannulas and Oxygen Masks Medical Device for Clinical Use
Tip: Sourcing certified medical devices with proper clinical documentation reduces audit risk and improves procurement speed.
1. Regulatory Compliance & Certifications for Sourcing Nasal Cannulas and Oxygen Masks
- ✔ CE Marking (EU)
- ✔ FDA 510(k) or PMA (US)
- ✔ ISO 13485 certification
2. Technical Suitability & Clinical Use
- ✔ Instruction manuals & datasheets
- ✔ Compatibility with clinical workflows
- ✔ Class I/II/III classification details
3. Supplier Experience & Support
- ✔ Hospital/clinic references
- ✔ On-site device training or support
- ✔ Regulatory audit history
4. Packaging, Storage & Logistics
Ensure sterile packaging and temperature-controlled shipping if required.
- ✔ Shelf life and handling guidelines
- ✔ Customs and import documentation
- ✔ Local distributor or service support
Pro Tip: Engage supplier formulation experts early — it can improve bioavailability and cut development time.
Conclusion
Sourcing Nasal Cannulas and Oxygen Masks as a medical device requires thorough certification review, supplier vetting, and logistics planning. With the right partner, you ensure compliance, safety, and clinical readiness.
Next Step: Get expert assistance in sourcing Nasal Cannulas and Oxygen Masks.
Request a Quote
Frequently Asked Questions For Sourcing of Nasal Cannulas and Oxygen Masks
What is the device classification of Nasal Cannulas and Oxygen Masks?
Nasal Cannulas and Oxygen Masks is classified as a
Class I/II/III medical device. This determines the level of regulatory control and testing required for approval and use.
What documentation is available with this device?
Each unit is shipped with full documentation, including the CE Certificate (if applicable), FDA clearance number, user manual, technical datasheet, and ISO 13485 certification (if applicable).
What is the typical lead time for Nasal Cannulas and Oxygen Masks?
Typical lead time is 2–4 weeks depending on stock availability, custom configurations, and shipping destination.
Does Nasal Cannulas and Oxygen Masks require special storage conditions?
No special storage required. Store in a clean, dry environment at room temperature unless otherwise specified.
Can Nasal Cannulas and Oxygen Masks be customized for specific clinical needs?
Yes, we offer customization options for bulk orders, including packaging, labeling, or technical features. Contact our team to discuss custom configurations or OEM partnerships.
This website uses cookies to ensure you get the best experience. By using our site, you agree to our
Privacy Policy.