How to Source Ciltacabtagene autoleucel infusion for Pharmaceutical Formulation
Ciltacabtagene autoleucel infusion (IV Infusion, Approved for relapsed/refractory multiple myeloma, with a targeted dose individualized based on patient weight) is classified under Cell & Gene Therapies. This guide highlights key sourcing factors buyers should consider when procuring high-quality Ciltacabtagene autoleucel infusion for formulation, R&D, or bulk manufacturing.
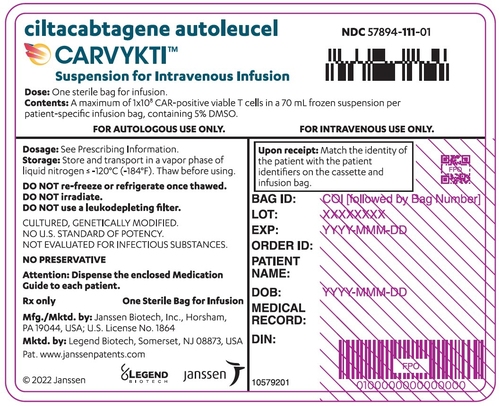
1. Regulatory Compliance & Documentation
Ensure suppliers provide:
- ✔ Valid DMF (Drug Master File)
- ✔ COA (Certificate of Analysis) for each batch
- ✔ GMP, ISO, or ICH Q7 compliance certificates
2. Purity, Grade & Specification Matching
Ciltacabtagene autoleucel infusion must meet exact grade and purity for your dosage form:
- ✔ USP / EP / JP grade verification
- ✔ Particle size distribution check
- ✔ Residual solvent and heavy metal limits
3. Supplier Reliability & Audit History
- ✔ Positive past audit reports
- ✔ Pharma client references
- ✔ Years of proven API manufacturing experience
4. Commercial Terms: Pricing, MOQ & Flexibility
- ✔ Transparent pricing
- ✔ Pilot batch MOQ flexibility
- ✔ Volume-based discounts
5. Lead Time, Inventory & Logistics
Typical lead time for Ciltacabtagene autoleucel infusion is 4–6 weeks.
- ✔ Emergency dispatch options
- ✔ Regional warehousing
- ✔ Special storage if needed
6. Formulation Support
- ✔ Technical datasheets
- ✔ Sample availability
- ✔ Stability study support
Conclusion
Sourcing Ciltacabtagene autoleucel infusion is more than procurement—it’s a strategic partnership. With its iv infusion form and Approved for relapsed/refractory multiple myeloma, with a targeted dose individualized based on patient weight specification, choosing a compliant supplier ensures consistent quality and regulatory approval. Prioritize documentation, verify compatibility, and build relationships with transparent, reliable suppliers to secure long-term success.
Frequently Asked Questions For Sourcing of Ciltacabtagene autoleucel infusion
What is the typical lead time for Ciltacabtagene autoleucel infusion?
Lead times range from 4–6 weeks depending on supplier and region.
Is Ciltacabtagene autoleucel infusion available in multiple grades?
Yes — common grades include USP, EP, and JP. Verify grade suitability for your dosage form before purchase.
Does Ciltacabtagene autoleucel infusion require special storage?
It should be stored in a cool, dry place away from direct sunlight.
Is a Drug Master File (DMF) available for Ciltacabtagene autoleucel infusion?
Yes, a DMF is available for regulated markets upon request. It includes detailed quality, manufacturing, and stability data.
Can I request samples or a pilot batch of Ciltacabtagene autoleucel infusion?
Yes — pilot batches or samples can be arranged for R&D, stability studies, or formulation trials. MOQ may vary based on region and regulatory scope.
What compliance certificates are available for Ciltacabtagene autoleucel infusion?
Available documentation may include GMP, ISO 9001, ISO 14001, and ICH Q7 certificates, along with CoA, TDS, and stability data.
Is Ciltacabtagene autoleucel infusion suitable for regulated markets like US/EU?
Yes — compliant with EU/US/WHO GMP standards and available with regulatory support documentation for filings.
Can Ciltacabtagene autoleucel infusion be used in fixed-dose combinations?
Yes — many formulations support inclusion in FDCs. Compatibility studies and formulation consultation are available on request.
